Topic 2: Atomic Structure & The Periodic Table
1. The Atom & Isotopes (2.1 - 2.5)
Atoms consist of a dense nucleus containing protons and neutrons, with electrons orbiting in shells.
| Particle | Relative Mass | Relative Charge |
|---|---|---|
| Proton | 1 | +1 |
| Neutron | 1 | 0 |
| Electron | 1/1840 | -1 |
- Atomic Number: Number of protons in the nucleus.
- Mass Number: Total number of protons and neutrons.
- Isotopes: Atoms of the same element with the same number of protons but different numbers of neutrons.
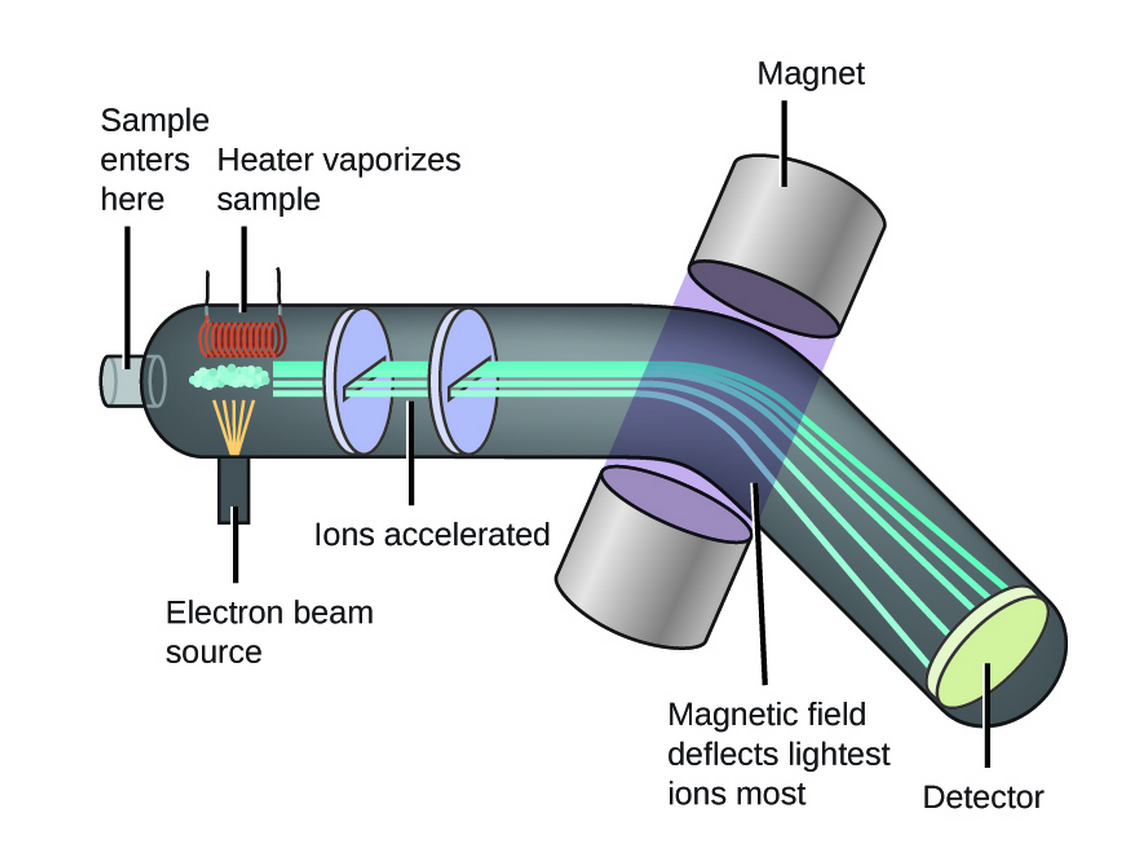
2. Mass Spectrometry (2.6 - 2.7)
Mass spectrometry is used to determine the isotopic composition of elements and the relative molecular mass of compounds.
Formula: Relative Atomic Mass ()
Diatomic Molecules (e.g. Chlorine)
For , with isotopes and in a 3:1 ratio, the mass spectrum shows peaks at 70, 72, and 74 in a 9:6:1 ratio.
3. Ionisation Energies (2.8 - 2.11)
First Ionisation Energy: The energy required to remove one mole of electrons from one mole of gaseous atoms.
Factors Affecting Ionisation Energy:
- Nuclear Charge: More protons = stronger attraction.
- Atomic Radius: Greater distance = weaker attraction.
- Shielding: More inner shells = weaker attraction.
4. Electronic Configuration (2.12 - 2.16)
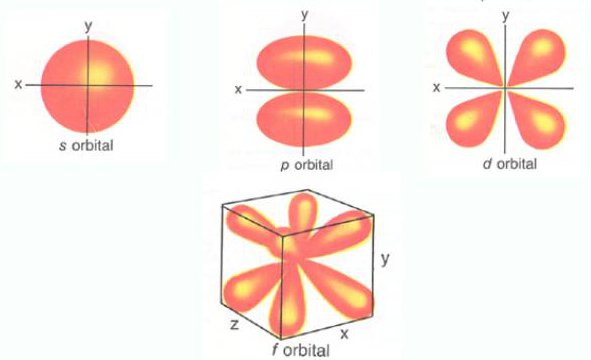
Electrons occupy orbitals (s, p, d). An orbital can hold two electrons with opposite spins.
- s-orbital: Spherical shape.
- p-orbital: Dumbbell shape.
Cr:
5. Periodicity (2.17 - 2.18)
Trends in melting and boiling temperatures are determined by structure and bonding.
- Giant Metallic (Li, Be, Na, Mg, Al): High melting points.
- Giant Covalent (C, Si): Very high melting points.
- Simple Molecular (P, S, Cl): Low melting points (dependent on London forces).
Practice Zone
Quick MCQ Practice
Q1: Which factor explains why the first ionisation energy decreases down Group 2?
- A) Increased nuclear charge
- B) Decreased shielding
- C) Increased atomic radius
- D) Decreased number of protons
Click for Answer
Correct Answer: C. As you go down a group, the outermost electron is further from the nucleus and experiences more shielding, making it easier to remove.
Exam-Style Calculation
Q2: A sample of Magnesium contains (79%), (10%), and (11%). Calculate the relative atomic mass.
Click for Worked Solution
Successive Ionisation Energy Challenge
Q3: An element in Period 3 has the following successive ionisation energies (kJ/mol): 738, 1451, 7733, 10543. Identify the element.
Click for Explanation
Answer: Magnesium (Mg). There is a massive jump between the 2nd and 3rd ionisation energies. This indicates the 3rd electron is being removed from a inner shell, meaning the element has 2 valence electrons (Group 2).